Introduction to Lars Medicare
Lars Medicare Pvt Ltd is a medical devices manufacturer focused on safe reliable and clinically relevant medical device solutions for hospitals and healthcare professionals across the world. From its advanced Sonipat plant in Haryana and with marketing offices in Delhi and Canada, Lars Medicare supports more than one hundred countries with high quality medical equipment and supplies for infusion therapy and critical care.
As a specialist in IV Cannula and medical disposable devices Lars Medicare understands how every medical device at the bedside must protect patient safety support clinical workflow and comply with strict medical device regulation in each market. Each product line is built to support nurses doctors and biomedical teams who rely on dependable medical equipment every day.
Company overview and global presence
Lars Medicare Pvt Ltd has grown into one of the trusted medical device companies from India with a strong reputation among medical equipment suppliers’ distributors and hospital groups. The company offers a focused portfolio of infusion device systems and related medical manufacturing solutions that cover the full IV therapy pathway.
The Sonipat plant is designed as a modern facility for medical device manufacturing with clean room environments-controlled processes and documented traceability. These systems support consistent quality for ISO certified medical devices and CE marked devices supplied to Europe Asia Africa Latin America and other regions through a strong network of medical device distributors and partners.
With a dedicated Delhi office for the Indian market and a Canada office supporting North America and nearby regions Lars Medicare handles global medical exports with localized service. The team works closely with medical equipment suppliers MedTech companies and group purchasing organizations to provide reliable product availability and technical support.
Why medical devices from Lars Medicare matter in modern care
Every medical device placed in a patient vein or connected to a therapy line becomes part of a complex clinical pathway. Poor quality devices can lead to leakage occlusion needlestick injuries infection risk and treatment delays. For this reason, hospitals look for medical devices manufacturer partners with proven track records in medical equipment manufacturing and continuous improvement.
Lars Medicare focuses on the safety and efficiency of infusion therapy. Each IV Cannula infusion device and connector is designed to support stable blood flow accurate drug delivery and secure fixation while considering daily nursing practice. Design choices account for vein preservation reduction of complications and overall patient comfort.
By combining engineering expertise with feedback from clinicians Lars Medicare aims to stand among the best medical device companies in the infusion segment. The goal is not only to deliver a single medical device but to ensure that all related medical equipment and supplies work together as a coherent system in the clinical environment.
Comprehensive portfolio of medical disposable devices
IV Cannula and Safety IV Cannula
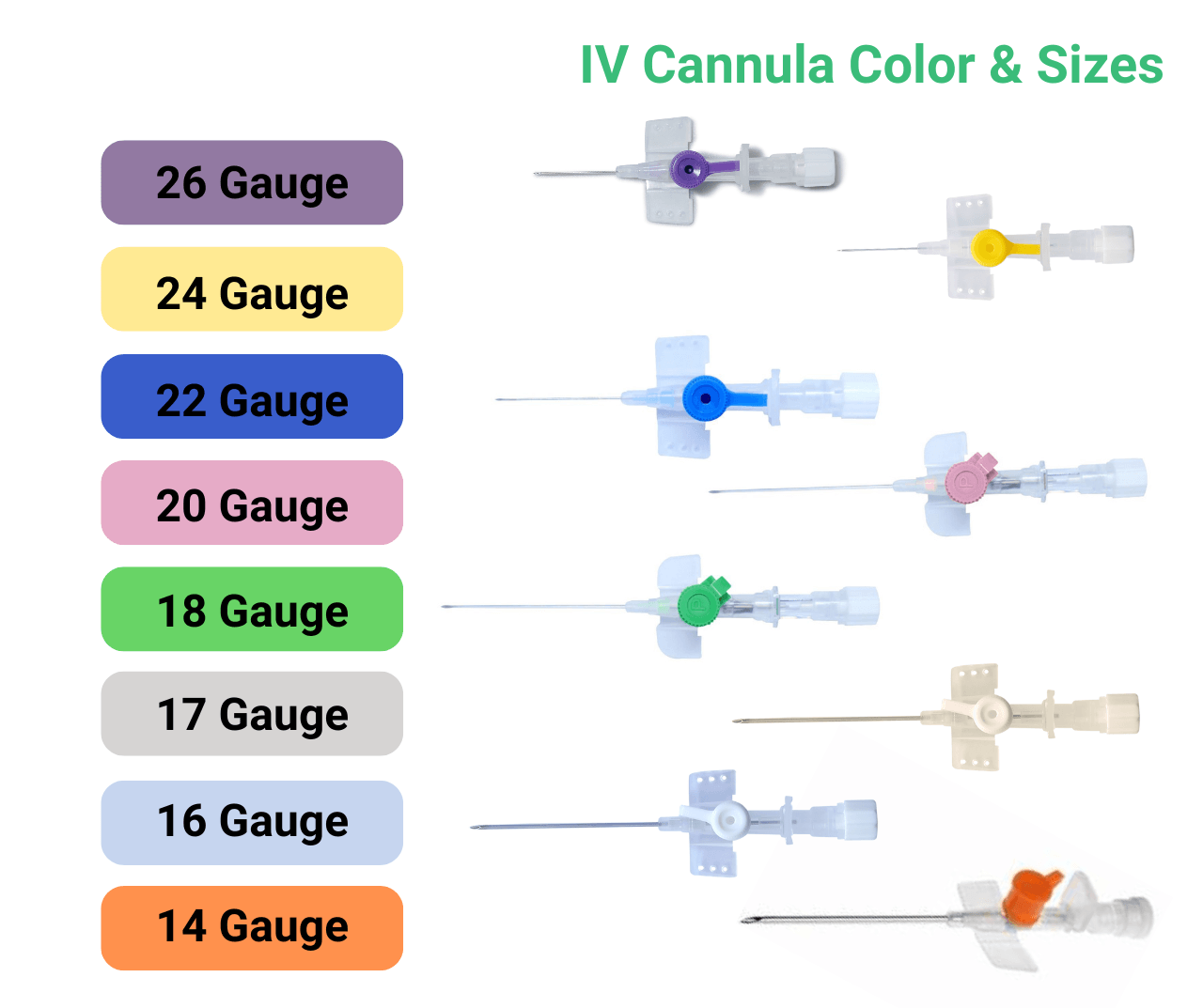
The core of the Lars Medicare portfolio is the IV Cannula range. Each IV Cannula is a single use medical device designed for peripheral venous access with smooth catheter material, kink resistance and atraumatic tip geometry. Colour coded sizes help clinicians match flow rate and vein size to the therapy protocol.
Safety IV Cannula options integrate mechanisms that help reduce accidental needlestick injuries for healthcare workers. These medical device variants often include safety clips or shielding features that activate after needle withdrawal. By promoting sharps safety Lars Medicare supports hospitals that follow international occupational health guidelines and medical device regulation focused on staff protection.
The IV Cannula portfolio is supported by clear labelling and user-friendly packaging so that medical equipment suppliers and hospital pharmacies can manage stock rotation easily.
Three Way Stop Cock and Three Way Stop Cock with Extension Tube
Three Way Stop Cock devices are vital at the bedside when clinicians need to connect multiple lines or sample blood while maintaining closed system integrity. Lars Medicare produces these components as precision moulded medical manufacturing products with smooth internal surfaces and leak proof connections.
The Three Way Stop Cock with Extension Tube combines a stop cock with a high-quality Extension Tube segment. This integrated medical device simplifies line management during infusion therapy reduces the need for multiple separate connectors and supports a tidy organized set up at the patient bedside. The Extension Tube is designed for flexibility transparency and kink resistance which supports visual inspection and reliable flow.
IV Extension Line and Extension Tube solutions
IV Extension Line products from Lars Medicare extend the reach of an IV set between the patient and the main infusion device or pump. Each IV Extension Line is a sterile medical device with secure luer connections and carefully selected tubing materials suitable for a wide range of medications and fluids.
Extension Tube solutions support various lengths and clinical needs. They are part of a wider ecosystem of medical equipment and supplies that help nurses position pumps and monitors in optimal locations while preserving patient comfort and mobility. When combined with IV Cannula and Needle Free Connector products the IV Extension Line offers a complete closed fluid pathway.
IV Flow Regulator and Infusion Set
The IV Flow Regulator is a critical medical device for manual control of flow rate without relying on electronic pumps. Lars Medicare designs each IV Flow Regulator to allow precise incremental adjustments with stable performance over time. Graduated markings assist clinicians in aligning flow rate with prescription requirements.
The standard Infusion Set range includes air vent options drip chambers sharp spikes and flexible tubing. Every Infusion Set is manufactured under strict process control at the Sonepat plant to reduce particulate contamination risk and ensure consistent drop size. These devices are compatible with a wide range of fluids and are essential components of daily medical equipment manufacturing output.
Blood Transfusion Set and Measured Volume Set
Blood products demand careful handling and a dedicated type of medical device. The Blood Transfusion Set from Lars Medicare is designed to meet transfusion guidelines with suitable filters smooth tubing and secure connections to both blood bags and IV access points. Transparent components allow visual monitoring during the transfusion process.
The Measured Volume Set is another key infusion device for pediatric and critical care environments. It allows accurate measurement of smaller fluid volumes which is essential for patients who require tight fluid balance. Each Measured Volume Set supports safe drug dilution and controlled delivery while integrating smoothly into existing medical equipment.
Needle Free Connector and closed system therapy
Needle Free Connector products help hospitals reduce the use of hypodermic needles for line access which decreases sharps related risk and supports infection prevention. The Needle Free Connector is a compact medical device that allows access with compatible syringes or IV sets while maintaining a closed pathway when not in use.
By using Needle Free Connector solutions in combination with IV Cannula IV Extension Line Infusion Set and IV Flow Regulator devices hospitals can build safer closed systems that support modern standards and align with current medical device regulation for infusion therapy.
Quality systems and regulatory alignment
Lars Medicare operates its Sonipat plant under a structured quality management system built around documented procedures validation plans and continuous process monitoring. These systems support ISO certified medical devices and CE marked devices supplied into regulated markets.
While individual product registrations are handled by local partners, the company maintains awareness of ISO 13485 principles as the global standard for medical device manufacturing quality systems. The quality team tracks updates in European medical device regulation and other regional frameworks so that product documentation, labeling and testing can support customers who work under these rules.
Quality control laboratories perform routine in process and final inspection on parameters such as leak integrity flow rate tensile strength and package integrity. Batch records and traceability data help medical device distributors and medical equipment suppliers meet their obligations for vigilance and field feedback.
By maintaining this disciplined approach to medical manufacturing, Lars Medicare strengthens its standing among medical device companies that focus on dependable and repeatable outcomes.
Manufacturing excellence at the Sonipat plant
The Sonepat plant is the heart of Lars Medicare’s operations. This facility brings together tooling, design, molding, extrusion assembly and packaging under a single integrated medical manufacturing site. Clean room assembly lines reduce contamination risk while automation supports consistent quality for each medical device.
Process engineers work closely with design teams to refine components such as IV Flow Regulator wheels stop cock handles and Needle Free Connector valves. Material selection focuses on biocompatibility clarity and mechanical strength. For every new medical device concept risk assessment and design verification support safe adoption at the bedside.
Because the full chain from raw material to finished device is controlled within the Sonepat plant Lars Medicare can respond quickly to customization requests and medical device contract manufacturing opportunities. This flexibility is important for MedTech companies and hospital groups that want private label versions or region specific configurations of IV Cannula Infusion Set or other devices.
Collaboration with medical equipment suppliers and distributors
Lars Medicare builds long term relationships with medical equipment suppliers and medical device distributors in more than one hundred countries. These partners rely on stable lead times, transparent documentation and technical training that explains how each medical device is intended to be used.
The Delhi office coordinates domestic supply to government hospitals private hospitals and corporate chains while the Canada office helps manage relationships in North America and nearby markets. Together with regional partners Lars Medicare supports tender’s framework contracts and customized packaging for different languages and regulatory needs.
By positioning itself as a flexible medical devices manufacturer with strong global medical exports the company provides distributors with a portfolio that can compete with many of the best medical device companies while delivering competitive value.
Support for hospitals clinicians and MedTech companies
For hospitals Lars Medicare offers more than individual medical equipment items. Clinical education materials demonstrate best practice for IV Cannula insertion maintenance flushing and replacement. Guidance is also available for integrating Infusion Set Blood Transfusion Set and Measured Volume Set products into existing protocols.
For MedTech companies seeking reliable medical device contract manufacturing partners Lars Medicare can provide design adaptation-controlled documentation and scale up support. The combination of technical expertise at the Sonepat plant and knowledge of medical equipment manufacturing standards allows efficient collaboration on new projects.
Biomedical engineers and procurement teams appreciate that every medical device is designed with maintainability and compatibility in mind. Standard connectors lengths and configurations help devices integrate smoothly into mixed vendor environments which is common in large hospitals.
Commitment to safety innovation and compliance
The ongoing evolution of medical device regulation requires manufacturers to plan for post market surveillance vigilance and life cycle management. Lars Medicare monitors field performance through feedback from distributors and hospitals and uses this information to improve future generations of IV Cannula Three Way Stop Cock IV Extension Line and other devices.
Innovation focuses on practical gains that matter to clinicians such as easier priming clearer drip chambers improved protective caps and smoother Needle Free Connector surfaces. Each small design change is reviewed against safety data and clinical input.
By aligning its culture with global expectations around quality ethics and transparency Lars Medicare aims to remain a trusted name in medical equipment and supplies. This approach supports hospitals that are building their own quality systems and wish to work with partners who share the same priorities.
Why choose Lars Medicare as your medical devices partner
Choosing a medical device supplier is a strategic decision that affects patient outcomes infection rates staff safety and budget stability. Lars Medicare stands out as a focused medical devices manufacturer for infusion therapy and related disposables with several advantages.
First the company offers a complete line that includes IV Cannula Safety IV Cannula Three Way Stop Cock Three Way Stop Cock with Extension Tube IV Extension Line Extension Tube IV Flow Regulator Infusion Set Blood Transfusion Set Measured Volume Set and Needle Free Connector products. This integrated range helps hospitals standardize on a single medical device brand for many infusion needs.
Second the Sonepat plant quality system combined with ISO certified medical devices and CE marked devices supports confidence in consistent performance. Awareness of iso13485 requirements and European medical device regulation helps global medical exports remain aligned with current expectations.
Third the presence of both the Delhi office and Canada office gives distributors and hospital groups clear regional points of contact. This structure supports responsive service and smooth communication for planning stocking and new product introductions.
Finally the long experience of Lars Medicare in medical manufacturing positions the company as a reliable partner for medical equipment manufacturing and contract projects with MedTech companies.
Conclusion
Lars Medicare Pvt Ltd has built its reputation as a dedicated medical devices manufacturer with a clear focus on infusion therapy safety and performance. From IV Cannula and Safety IV Cannula to IV Flow Regulator, IV Infusion Set, Blood Transfusion Set Measured Volume Set IV Extension Line Extension Tube and Needle Free Connector devices every product reflects careful engineering and process control at the Sonepat plant.
Supported by ISO certified medical devices, CE-marked devices, and a strong culture of quality, the company serves hospitals, medical equipment suppliers, and medical device distributors in more than one hundred countries. With marketing support from the Delhi office and Canada office, Lars Medicare is well placed to expand its global medical exports and partnerships. Healthcare organizations searching for reliable medical device partners can look to Lars Medicare as a company that combines manufacturing excellence, regulatory awareness and clinical understanding. By choosing Lars Medicare hospitals and distributors gain access to a coherent portfolio of medical equipment and supplies designed to support safe effective and efficient patient care in every setting.