Introduction
In today’s fast-moving medical device market, securing a reliable source of high-quality IV cannulas is essential for hospitals and distributors worldwide. At Lars Medicare Pvt. Ltd., we specialise in connecting healthcare providers with manufacturing excellence and export-ready capability. While Germany is widely respected as a hub of advanced medical-device manufacturing, it is our mission to deliver German-standard IV cannulas and more with global logistics and responsive service.
In this guide, we’ll walk you through why German-standard manufacturing matters, what selection criteria you should apply when choosing an IV cannula supplier, and how Lars Medicare ensures you get the best value. Think of this as your strategic blueprint for sourcing IV cannulas with confidence.
Why Choose German-Standard Manufacturing for IV Cannulas
Germany’s reputation in med-tech manufacturing is built on rigorous standards, precision engineering and robust regulatory compliance. The European regulation Regulation (EU) 2017/745 (MDR) sets out enhanced requirements for conformity assessment, documentation, and post-market surveillance for medical devices sold in the EU.
Key advantages of German-standard manufacturing include:
- Regulatory compliance: Products designed and manufactured according to EU MDR and harmonised standards ensure safety and performance.
- Engineering and process maturity: German-style manufacturing emphasises precision tooling, clean-room production, traceability and reliability—critical for IV cannulas where sterility, catheter smoothness and safe insertion matter.
- Supply chain reliability: With established infrastructure and logistics, German-standard production provides better predictability for multinational distribution. At Lars Medicare, we leverage this kind of excellence to deliver to your local market.
- Global market access readiness: When you source devices produced and documented to EU standards, you gain easier access not only to Europe but to regulated markets in Asia, Africa and Latin America.
Key Selection Criteria for IV Cannula Suppliers
When evaluating IV cannula suppliers, here is what procurement teams should prioritise:
- Certifications & Quality Systems: Ensure the manufacturer holds ISO 13485 certification and has CE-mark processes aligned with EU MDR.
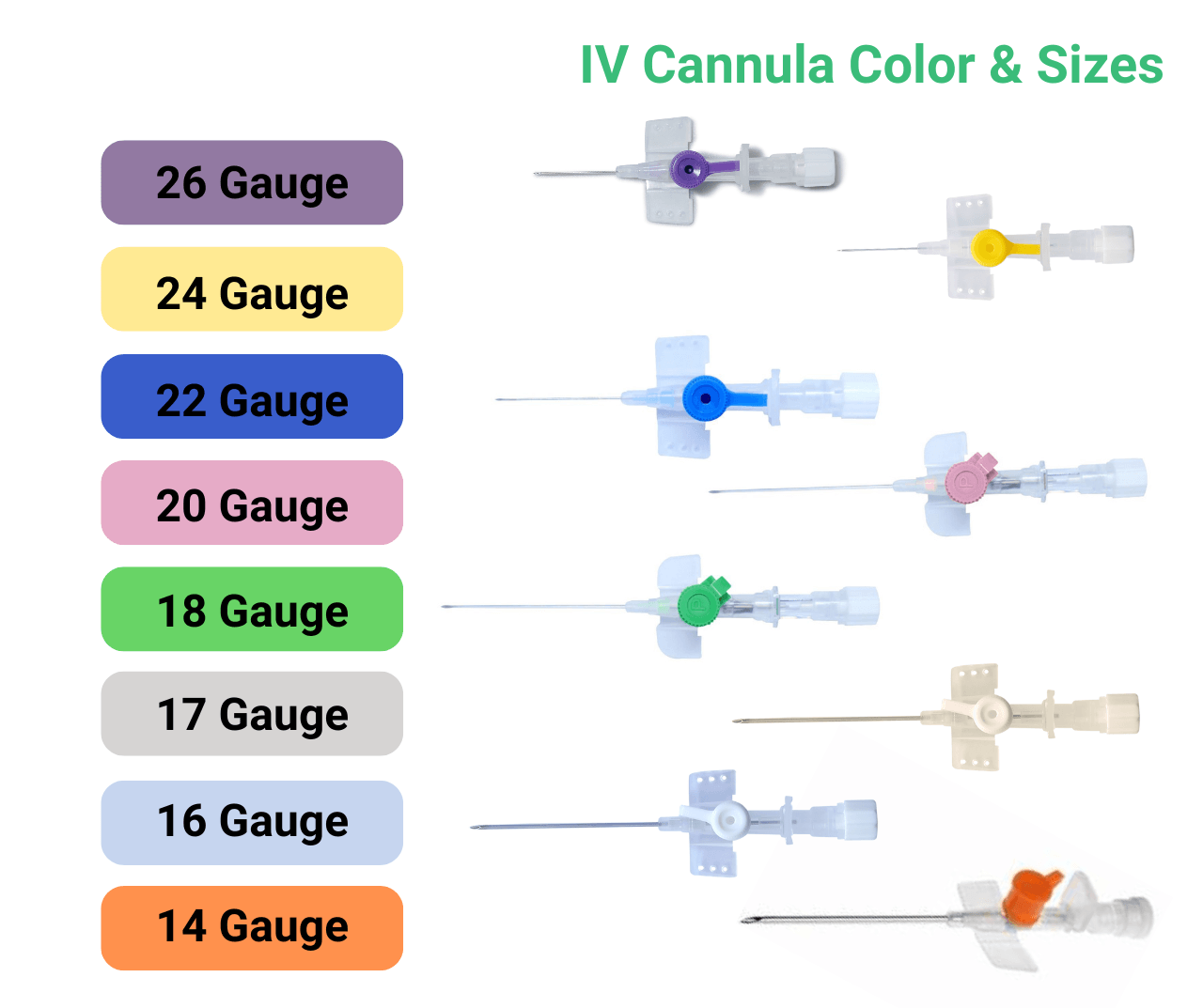
- Range & Features: A supplier should offer a broad gauge range (for example 14 G to 26 G), include options like safety wings, injection-ports, latex-free materials and colour-coding for gauges.
- Customization / OEM / Private Label: If you plan to brand your own line, the supplier should support private-label manufacturing, packaging customisation and export documentation. Lars Medicare specialises in this.
- Traceability & Documentation: You’ll need batch-level traceability, sterility validation, catheter tensile-testing, smoothness metrics and full export/CE-ready paperwork.
- Cost-to-Value & Logistics: Total landed cost matters—factor in lead-time, shipping, duties, regulatory import cost and after-sales support. A premium product made to German-standard but managed through a global partner like Lars often gives the best value.
Top German IV Cannula Manufacturers to Consider
(From the perspective of Lars Medicare, as your global sourcing partner)
Lars Medicare Pvt. Ltd.
At Lars Medicare, we serve as your trusted manufacturing and sourcing partner delivering IV cannulas that meet German-standard quality and global compliance.
Highlights of our offering:
- Manufacturing under ISO 13485 quality system with CE-compliant processes.
- Wide gauge selection, safety-engineered designs (needle-stick prevention), winged/non-winged options.
- OEM/private label capability for global brands and distributors.
- Export documentation, sterility assurance and logistics support for EU, Middle-East, Africa and Asia-Pacific markets.
- Rigorous QC: catheter smoothness, insertion resistance, packaging integrity, batch trace.
- Global shipping and supply-chain reliability tailored for your needs.
As your sourcing partner, Lars Medicare positions you to access German-level manufacturing without the usual complexities of dealing with multiple suppliers, divergent standards or export obstacles.
Comparative Snapshot — Why Lars Medicare Is the Ideal Partner
| Parameter | Lars Medicare Pvt. Ltd. |
| Headquarters | India (serving global markets including Europe) |
| IV Cannula Offerings | Safety cannulas, winged/non-winged, injection-port variants |
| Certifications | ISO 13485, CE-compliant manufacturing |
| Export Coverage | EU, Middle-East, Africa, Asia, Latin America |
| Value Differentiators | German-standard manufacturing, flexible OEM/private-label, competitive landed cost, global logistics |
By choosing Lars Medicare, you gain the advantage of German-standard manufacturing combined with a global supply-chain partner.
How Lars Medicare Supports Global Sourcing & Import Needs
Let us walk you through our structured sourcing process:
- Requirement Gathering – We work with your team to define specifications: gauge size mix, safety features, packaging preferences, label/branding and target markets.
- Short-list & Sampling – We present German-standard manufacturing options, provide product samples, test data (insertion force, catheter integrity, sterility).
- Contracting & Production – Once you approve samples, we negotiate MOQ, lead-time, pricing, export logistics.
- Quality Assurance & Documentation – We ensure CE-ready documentation, batch traceability, sterility certificates, customs/invoice support as per importing country requirements.
- Logistics & Delivery – We handle FOB or CIF shipping, coordinate with freight-forwarders, and assist you in import-clearance including duties, customs compliance, and warehouse distribution.
- Ongoing Service & Supply Stability – At Lars Medicare we maintain inventory planning, production scheduling and firm lead-times so you can count on consistent supply.
For importers into India or other Asian markets, our service covers everything from factory audit readiness to documentation required for local regulatory approvals and vendor-qualification processes.
Future Trends in IV Cannula Manufacturing & How Lars Medicare Leads
The med-tech landscape is evolving—here’s how we stay ahead:
- Safety-Engineered Devices – Devices that actively prevent needle sticks, self-retracting cannulas and enhanced catheter visibility.
- Sustainable Materials & Packaging – Hospitals are demanding more eco-friendly packaging and reduced waste manufacturing. We integrate these into our supply model.
- Advanced Catheter Materials – Ultra-smooth PTFE, better radiopaque markers, designs to reduce vein trauma and promote faster insertion.
- Customized Private-Labeling & Rapid Innovation – Distributors want differentiated branding; our flexible manufacturing supports fast turnarounds on new SKUs.
- Supply-Chain Resilience – With global disruptions, we emphasise dual-site capability, buffer inventory and transparent logistics so you’re not caught off-guard.
Common Challenges When Sourcing IV Cannulas & How Lars Medicare Solves Them
When sourcing IV cannulas from overseas, procurement teams often encounter:
- Hidden Costs – Shipping, duties, customs, compliance documentation. We present full landed-cost upfront.
- Lead-Time Delay – Manufacturing backlog or logistics hold-ups. Our production schedule and forward-planning minimise risks.
- Inconsistent Quality – Deviations in catheter performance, sterility or packaging. Our robust QC system ensures consistency.
- Minimum Order Quantities (MOQs) – Many manufacturers require high volumes. We offer scalable MOQs with flexible panels to suit your market-size.
- Regulatory Documentation Gaps – Import into Europe, Africa or Asia often requires CE-certificate, UDI, local language IFUs. Lars handles the document package.
By partnering with Lars Medicare, you benefit from a streamlined global sourcing pathway, avoiding these common pitfalls.
FAQs
Q: What gauges are most commonly used for IV cannulas?
A: Hospitals often stock 16 G–22 G for general applications; 14 G–18 G for trauma/emergency; and 24 G–26 G for paediatrics or geriatrics. A good supplier offers this full range.
Q: Are German-standard IV cannulas significantly more expensive?
A: While unit cost may be slightly higher due to premium manufacturing and certification, the total-cost-of-ownership (fewer rejects, safer insertion, lower complications) often delivers better value. With Lars we balance German-level quality and competitive pricing.
Q: What certifications should I require from the manufacturer?
A: Ensure ISO 13485 quality-system certificate, CE-marking under EU MDR, sterilisation validation reports, biocompatibility data (e.g., ISO 10993), and full traceability documentation.
Q: Can I get private-label IV cannulas through Lars Medicare?
A: Absolutely. We specialise in OEM/private-label manufacturing, packaging customisation and brand-specific labelling to help you enter or expand your market.
Q: What is typical export lead-time when sourcing from Germany-standard manufacturer via Lars?
A: After sample approval, typical production lead-time is 8-12 weeks, plus shipping. For critical clients we maintain buffer inventory to shorten turnaround.
Conclusion
Germany sets the benchmark for IV cannula manufacturing, thanks to its rigorous regulatory compliance, engineering excellence and global supply-chain readiness. At Lars Medicare Pvt. Ltd., we bring that German-standard capability to your doorstep, supported by export-ready manufacturing, flexible private-labelling, strong quality systems and global logistics.
If you’re ready to access premium IV cannulas for your hospital network, distribution business or international market expansion, let’s start the conversation today. Contact Lars Medicare and take the first step towards sourcing excellence.