Needle Safety Beyond the Lock: Why Sterilization Validation is the Importer’s Most Critical Quality Check
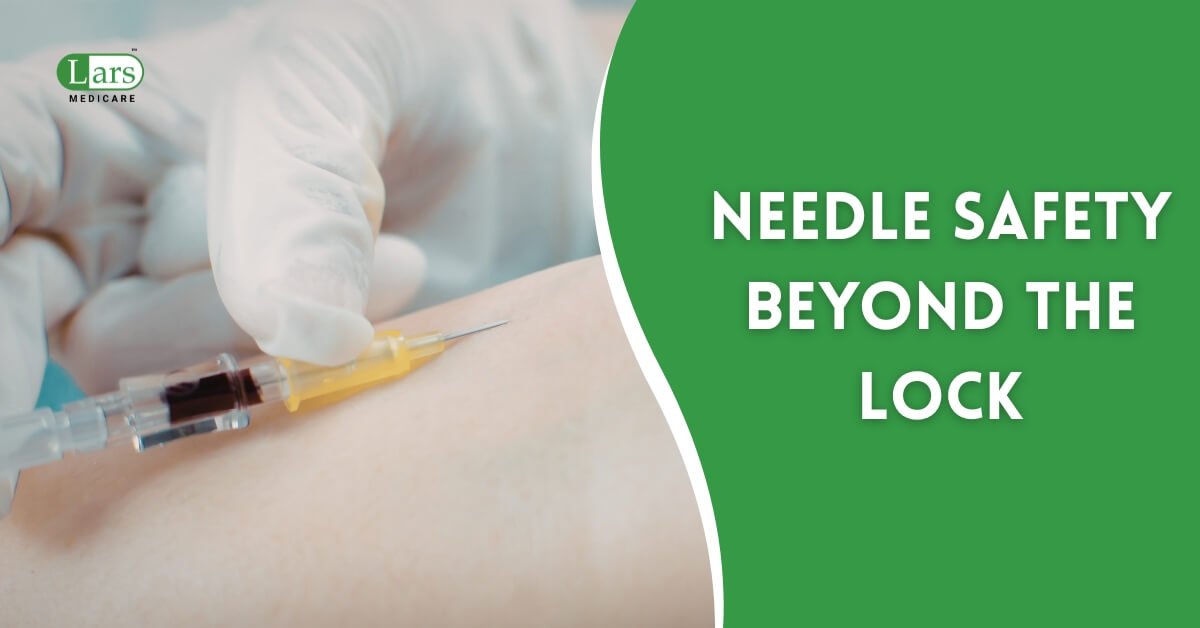
Every procurement manager knows to check for the visible safety mechanism on an IV Cannula. But for bulk medical devices, the single greatest point of failure – and the highest liability risk – is often hidden deep within the manufacturing process: Sterilization Validation. If a sterile-labeled device is compromised, it is not merely a clinical […]
